How Much Money Does Glazosmithkline Make On Thorazine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Hibernal, Largactil, Thorazine, Sonazine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682040 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets and syrup available), rectal, intramuscular (IM), intravenous infusion (Four) |
| Drug class | Typical antipsychotic |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10–lxxx% (Oral; large interindividual variation)[three] |
| Protein binding | 90–99%[3] |
| Metabolism | Liver, mostly CYP2D6-mediated[three] |
| Elimination half-life | xxx hours[four] |
| Excretion | Urine (43–65% in 24 hrs)[three] |
| Identifiers | |
| IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.000.042 |
| Chemic and physical data | |
| Formula | C 17 H nineteen Cl N 2 S |
| Molar mass | 318.86 1000·mol−ane |
| 3D model (JSmol) |
|
| SMILES
| |
| InChI
| |
| (verify) | |
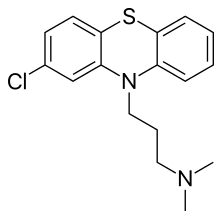
Chlorpromazine (CPZ), marketed nether the brand names Thorazine and Largactil amid others, is an antipsychotic medication.[four] It is primarily used to treat psychotic disorders such equally schizophrenia.[iv] Other uses include the handling of bipolar disorder, severe behavioral problems in children including those with attending arrears hyperactivity disorder, nausea and vomiting, anxiety before surgery, and hiccups that do not ameliorate following other measures.[4] It tin can be given by oral fissure, by injection into a muscle, or into a vein.[4]
Chlorpromazine is in the typical antipsychotic class,[four] and, chemically, is i of the phenothiazines. Its mechanism of activeness is not entirely articulate but believed to be related to its ability as a dopamine adversary.[4] It as well has anti-serotonergic and antihistaminergic properties.[4]
Common side furnishings include movement problems, sleepiness, dry mouth, low blood force per unit area upon standing, and increased weight.[4] Serious side furnishings may include the potentially permanent motion disorder tardive dyskinesia, neuroleptic malignant syndrome, severe lowering of the seizure threshold, and low white blood cell levels.[four] In older people with psychosis as a result of dementia it may increase the risk of expiry.[four] It is unclear if it is safe for employ in pregnancy.[4]
Chlorpromazine was developed in 1950 and was the first antipsychotic.[5] [vi] It is on the World Health Organization'south List of Essential Medicines.[seven] Its introduction has been labeled as one of the smashing advances in the history of psychiatry.[viii] [9] It is available as a generic medication.[four]
Medical uses [edit]
Chlorpromazine is used in the handling of both astute and chronic psychoses, including schizophrenia and the manic phase of bipolar disorder, as well as amphetamine-induced psychosis.
In a 2013 comparing of 15 antipsychotics in schizophrenia, chlorpromazine demonstrated balmy-standard effectiveness. It was 13% more effective than lurasidone and iloperidone, approximately as effective as ziprasidone and asenapine, and 12–sixteen% less effective than haloperidol, quetiapine, and aripiprazole.[10]
A 2014 systematic review carried out by Cochrane included 55 trials that compared the effectiveness of chlorpromazine versus placebo for the treatment of schizophrenia. Compared to the placebo grouping, patients nether chlorpromazine experienced less relapse during 6 months to 2 years follow-upwardly. No difference was found between the two groups beyond two years of follow-upwards. Patients under chlorpromazine showed a global improvement in symptoms and functioning. The systematic review also highlighted the fact that the side effects of the drug were 'severe and debilitating', including sedation, considerable weight gain, a lowering of claret pressure, and an increased risk of suffering from acute movement disorders. They too noted that the quality of evidence of the 55 included trials was very low and that 315 trials could not be included in the systematic review due to their poor quality. They called for further inquiry on the subject area, equally chlorpromazine is a inexpensive benchmark drug and one of the nearly used treatments for schizophrenia worldwide. [eleven]
Chlorpromazine has also been used in porphyria and equally role of tetanus treatment. It nonetheless is recommended for short-term management of severe feet and psychotic aggression. Resistant and severe hiccups, severe nausea/emesis, and preanesthetic conditioning are other uses.[12] [13] Symptoms of delirium in hospitalized AIDS patients accept been effectively treated with depression doses of chlorpromazine.[14]
Other [edit]
Chlorpromazine is occasionally used off-label for handling of astringent migraine.[15] [xvi] It is often, particularly every bit palliation, used in pocket-size doses to reduce nausea suffered by opioid-treated cancer patients and to intensify and prolong the analgesia of the opioids as well.[fifteen] [17] Efficacy has been shown in treatment of symptomatic hypertensive emergency.
In Germany, chlorpromazine even so carries label indications for insomnia, astringent pruritus, and preanesthesia.[18]
Chlorpromazine and other phenothiazines have been demonstrated to possess antimicrobial properties, but are not currently used for this purpose except for a very small number of cases.
| Measured effect | Findings summary | Findings range | Quality of evidence |
|---|---|---|---|
| Global furnishings | |||
| Non whatever improvement (ix weeks – 6 months) | 30% less risk of having no improvement in mental state, behaviour and functioning | RR 0.7 CI 0.6 to 0.9 | Very low (judge of issue uncertain) |
| Relapse (vi months – 2 years) | 35% less chance of relapse | RR 0.7 CI 0.5 to 0.ix | |
Adverse effects [edit]
There appears to be a dose-dependent risk for seizures with chlorpromazine treatment.[xx] Tardive dyskinesia (involuntary, repetitive body movements) and akathisia (a feeling of inner restlessness and inability to stay however) are less ordinarily seen with chlorpromazine than they are with high potency typical antipsychotics such as haloperidol[21] or trifluoperazine, and some bear witness suggests that, with conservative dosing, the incidence of such furnishings for chlorpromazine may be comparable to that of newer agents such as risperidone or olanzapine.[22]
Chlorpromazine may eolith in ocular tissues when taken in loftier dosages for long periods of time.
| Measured outcome | Findings summary | Findings range | Quality of evidence |
|---|---|---|---|
| Agin effects | |||
| Weight gain | 5 times more probable to have considerable weight gain, around 40% with chlorpromazine gaining weight | RR 4.9 CI two.3 to 10.4 | Very low (estimate of effect uncertain) |
| Sedation | 3 times more probable to cause sedation, around 30% with chlorpromazine | RR 2.8 CI 2.3 to 3.five | |
| Acute move disorder | iii.5 times more than likely to cause easily reversible but unpleasant severe stiffening of muscles, effectually 6% with chlorpromazine | RR 3.5 CI one.5 to viii.0 | |
| Parkinsonism | 2 times more likely to cause parkinsonism (symptoms such as tremor, hesitancy of movement, decreased facial expression), around 17% with chlorpromazine | RR ii.1 CI ane.half-dozen to 2.eight | |
| Decreased blood pressure with dizziness | 3 times more probable to crusade decreased blood pressure and dizziness, around 15% with chlorpromazine | RR two.four CI 1.vii to 3.3 | |
Contraindications [edit]
Accented contraindications include:[3]
- Circulatory depression
- CNS depression
- Blackout
- Drug intoxication
- Os marrow suppression
- Phaeochromocytoma
- Hepatic failure
- Active liver affliction
- Previous hypersensitivity (including jaundice, agranulocytosis, etc.) to phenothiazines, especially chlorpromazine, or any of the excipients in the formulation beingness used.
Relative contraindications include:[3]
- Epilepsy
- Parkinson'southward affliction
- Myasthenia gravis
- Hypoparathyroidism
- Prostatic hypertrophy
Very rarely, elongation of the QT interval may occur, increasing the risk of potentially fatal arrhythmias.[23]
Interactions [edit]
Consuming food prior to taking chlorpromazine orally limits its absorption; likewise, cotreatment with benztropine can as well reduce chlorpromazine assimilation.[3] Alcohol can also reduce chlorpromazine absorption.[three] Antacids wearisome chlorpromazine absorption.[three] Lithium and chronic treatment with barbiturates tin increase chlorpromazine clearance significantly.[3] Tricyclic antidepressants (TCAs) tin decrease chlorpromazine clearance and hence increase chlorpromazine exposure.[3] Cotreatment with CYP1A2 inhibitors like ciprofloxacin, fluvoxamine or vemurafenib can reduce chlorpromazine clearance and hence increase exposure and potentially likewise adverse furnishings.[3] Chlorpromazine tin can also potentiate the CNS depressant effects of drugs like barbiturates, benzodiazepines, opioids, lithium and anesthetics and hence increase the potential for adverse effects such as respiratory depression and sedation.[three]
Chlorprozamine is too a moderate inhibitor of CYP2D6 and a substrate for CYP2D6, and hence tin inhibit its own metabolism.[12] Information technology tin can also inhibit the clearance of CYP2D6 substrates such as dextromethorphan, potentiating their effects.[12] Other drugs similar codeine and tamoxifen, which require CYP2D6-mediated activation into their respective active metabolites, may have their therapeutic furnishings adulterate.[12] Likewise, CYP2D6 inhibitors such as paroxetine or fluoxetine can reduce chlorpromazine clearance, increasing serum levels of chlorpromazine and potentially its adverse effects.[3] Chlorpromazine as well reduces phenytoin levels and increases valproic acid levels.[3] Information technology also reduces propranolol clearance and antagonizes the therapeutic effects of antidiabetic agents, levodopa (a Parkinson's medication. This is likely due to the fact that chlorpromazine antagonizes the Dtwo receptor which is ane of the receptors dopamine, a levodopa metabolite, activates), amphetamines and anticoagulants.[3] It may also interact with anticholinergic drugs such as orphenadrine to produce hypoglycaemia (depression claret saccharide).[3]
Chlorpromazine may also interact with epinephrine (adrenaline) to produce a paradoxical fall in claret pressure level.[3] Monoamine oxidase inhibitors (MAOIs) and thiazide diuretics may also accentuate the orthostatic hypotension experienced past those receiving chlorpromazine treatment.[3] Quinidine may interact with chlorpromazine to increment myocardial low.[3] Also, it may too antagonize the effects of clonidine and guanethidine.[3] It also may reduce the seizure threshold and hence a corresponding titration of anticonvulsant treatments should be considered.[3] Prochlorperazine and desferrioxamine may as well interact with chlorpromazine to produce transient metabolic encephalopathy.[3]
Other drugs that prolong the QT interval, such as quinidine, verapamil, amiodarone, sotalol and methadone, may also interact with chlorpromazine to produce additive QT interval prolongation.[three]
Discontinuation [edit]
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[24] Symptoms of withdrawal unremarkably include nausea, vomiting, and loss of appetite.[25] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[25] Less commonly, there may be a feeling of the globe spinning, numbness, or muscle pains.[25] Symptoms generally resolve after a short period of fourth dimension.[25]
At that place is tentative evidence that discontinuation of antipsychotics tin can result in psychosis.[26] It may besides result in reoccurrence of the condition that is existence treated.[27] Rarely, tardive dyskinesia tin can occur when the medication is stopped.[25]
Pharmacology [edit]
Chlorpromazine is classified as a low-say-so typical antipsychotic. Low-potency antipsychotics have more anticholinergic side furnishings, such as dry mouth, sedation, and constipation, and lower rates of extrapyramidal side effects, while high-potency antipsychotics (such as haloperidol) have the reverse contour.[12]
Pharmacokinetics [edit]
| Bioavailability | tmax | CSS | Protein bound | Vd | t1/ii | Details of metabolism | Excretion | Notes |
|---|---|---|---|---|---|---|---|---|
| 10–80% | 1–4 hours (Oral); half-dozen–24 hours (IM) | 100–300 ng/mL | ninety–99% | 10–35 L/kg (mean: 22 50/kg) | 30±7 hours | CYP2D6, CYP1A2—mediated into over 10 major metabolites.[12] The major routes of metabolism include hydroxylation, N-oxidation, sulfoxidation, demethylation, deamination and conjugation. There is piddling evidence supporting the evolution of metabolic tolerance or an increase in the metabolism of chlorpromazine due to microsomal liver enzymes following multiple doses of the drug.[29] | Urine (43–65% afterwards 24 hours) | Its loftier degree of lipophilicity (fatty solubility) allows it to be detected in the urine for up to 18 months.[3] [thirty] Less than ane% of the unchanged drug is excreted via the kidneys in the urine, in which 20–70% is excreted as conjugated or unconjugated metabolites, whereas 5–six% is excreted in feces.[xxx] |
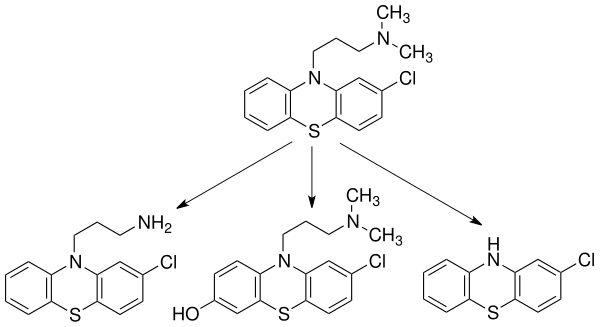
Three common metabolites of chlorpromazine
Pharmacodynamics [edit]
| Site | Ki | Species | Ref |
|---|---|---|---|
| 5-HT1A | 3115 | Human | [31] |
| 5-HT1B | 1,489 | Human | [32] |
| 5-HT1D | 452 | Human | [32] |
| 5-HT1E | 344 | Human | [32] |
| 5-HT2A | 2.75 | Homo | [33] |
| 5-HT2C | 25 | Homo | [34] |
| 5-HT3 | 776 | Homo | [35] |
| 5-HT5A | 118 | Human | [32] |
| five-HT6 | nineteen.v | Human | [35] |
| 5-HT7 | 21 | Human | [32] |
| α1A | 0.28 | Human | [32] |
| α1B | 0.81 | Human | [32] |
| α2A | 184 | Human | [32] |
| α2B | 28 | Homo | [32] |
| α2C | 46 | Homo | [32] |
| β1 | >10,000 | Human being | [32] |
| βii | >10,000 | Human | [32] |
| G1 | 47 | Human | [32] |
| Thou2 | 433 | Human | [32] |
| M3 | 47 | Homo | [32] |
| M4 | 151 | Human | [32] |
| Done | 114.viii | Human being | [35] |
| D2 | vii.244 | Human | [35] |
| D3 | 6.9 | Human | [36] |
| D4 | 32.36 | Human | [35] |
| H1 | iv.25 | Human being | [36] |
| Htwo | 174 | Human | [32] |
| H3 | 1,000 | Human | [36] |
| H4 | 5,048 | Human | [32] |
| NET | 2,443 | Human | [32] |
| DAT | >10,000 | Man | [32] |
Chlorpromazine is a very effective antagonist of D2 dopamine receptors and similar receptors, such equally D3 and D5. Unlike well-nigh other drugs of this genre, information technology also has a high affinity for D1 receptors. Blocking these receptors causes diminished neurotransmitter binding in the forebrain, resulting in many different effects. Dopamine, unable to demark with a receptor, causes a feedback loop that causes dopaminergic neurons to release more dopamine. Therefore, upon first taking the drug, patients will experience an increase in dopaminergic neural activeness. Eventually, dopamine production of the neurons will drop substantially and dopamine will be removed from the synaptic cleft. At this bespeak, neural action decreases greatly; the continual blockade of receptors only compounds this effect.[12]
Chlorpromazine acts equally an antagonist (blocking agent) on unlike postsynaptic and presynaptic receptors:
- Dopamine receptors (subtypes Di, D2, Diii and D4), which account for its dissimilar antipsychotic properties on productive and unproductive symptoms, in the mesolimbic dopamine organization accounts for the antipsychotic effect whereas the blockade in the nigrostriatal arrangement produces the extrapyramidal furnishings
- Serotonin receptors (five-HTii, 5-HT6 and 5-HT7), with anxiolytic, antidepressant and antiaggressive properties equally well as an attenuation of extrapyramidal side furnishings, but likewise leading to weight gain and ejaculation difficulties.
- Histamine receptors (H1 receptors, bookkeeping for sedation, antiemetic effect, vertigo, and weight proceeds)
- α1- and α2-adrenergic receptors (accounting for sympatholytic properties, lowering of blood force per unit area, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence also every bit sexual dysfunction, just may besides attenuate pseudoparkinsonism – controversial. Also associated with weight gain as a result of blockage of the adrenergic alpha 1 receptor)
- Mone and K2 muscarinic acetylcholine receptors (causing anticholinergic symptoms such equally dry out mouth, blurred vision, constipation, difficulty or disability to urinate, sinus tachycardia, electrocardiographic changes and loss of memory, but the anticholinergic activeness may attenuate extrapyramidal side effects).
The presumed effectiveness of the antipsychotic drugs relied on their power to block dopamine receptors. This assumption arose from the dopamine hypothesis that maintains that both schizophrenia and bipolar disorder are a outcome of excessive dopamine activity. Furthermore, psychomotor stimulants similar cocaine that increase dopamine levels can crusade psychotic symptoms if taken in excess.[37]
Chlorpromazine and other typical antipsychotics are primarily blockers of D2 receptors. In fact an nigh perfect correlation exists between the therapeutic dose of a typical antipsychotic and the drug'southward analogousness for the D2 receptor. Therefore, a larger dose is required if the drug's affinity for the D2 receptor is relatively weak. A correlation exists betwixt average clinical authorization and analogousness of the antipsychotics for dopamine receptors.[38] Chlorpromazine tends to have greater outcome at serotonin receptors than at D2 receptors, which is notably the opposite effect of the other typical antipsychotics. Therefore, chlorpromazine with respect to its effects on dopamine and serotonin receptors is more like to the singular antipsychotics than to the typical antipsychotics.[38]
Chlorpromazine and other antipsychotics with sedative properties such as promazine and thioridazine are amid the most potent agents at α-adrenergic receptors. Furthermore, they are also amidst the near potent antipsychotics at histamine H1 receptors. This finding is in agreement with the pharmaceutical development of chlorpromazine and other antipsychotics as anti-histamine agents. Furthermore, the brain has a college density of histamine H1 receptors than any body organ examined which may account for why chlorpromazine and other phenothiazine antipsychotics are as stiff at these sites as the about strong classical antihistamines.[39]
In addition to influencing the neurotransmitters dopamine, serotonin, epinephrine, norepinephrine, and acetylcholine it has been reported that antipsychotic drugs could achieve glutamatergic effects. This mechanism involves straight effects on antipsychotic drugs on glutamate receptors. By using the technique of functional neurochemical assay chlorpromazine and phenothiazine derivatives have been shown to have inhibitory effects on NMDA receptors that appeared to be mediated by action at the Zn site. It was found that there is an increment of NMDA action at low concentrations and suppression at loftier concentrations of the drug. No meaning divergence in glutamate and glycine activity from the effects of chlorpromazine were reported. Farther work will exist necessary to determine if the influence in NMDA receptors by antipsychotic drugs contributes to their effectiveness.[40]
Chlorpromazine does too act as a FIASMA (functional inhibitor of acid sphingomyelinase).[41]
Peripheral furnishings [edit]
Chlorpromazine is an antagonist to H1 receptors (provoking antiallergic effects), H2 receptors (reduction of forming of gastric juice), Mane and G2 receptors (dry mouth, reduction in forming of gastric juice) and some 5-HT receptors (different anti-allergic/gastrointestinal actions).
Because it acts on and then many receptors, chlorpromazine is frequently referred to as a "dirty drug".[42]
History [edit]

Ad for Thorazine (chlorpromazine) from the early 1960s[43]
In 1933, the French pharmaceutical visitor Laboratoires Rhône-Poulenc began to search for new anti-histamines. In 1947, it synthesized promethazine, a phenothiazine derivative, which was found to take more than pronounced sedative and antihistaminic furnishings than earlier drugs.[44] A yr later, the French surgeon Pierre Huguenard used promethazine together with pethidine as part of a cocktail to induce relaxation and indifference in surgical patients. Another surgeon, Henri Laborit, believed the compound stabilized the central nervous organization past causing "artificial hibernation", and described this country every bit "sedation without narcosis". He suggested to Rhône-Poulenc that they develop a compound with better stabilizing properties.[45] In December 1950, the chemist Paul Charpentier produced a series of compounds that included RP4560 or chlorpromazine.[five] Simone Courvoisier conducted behavioural tests and constitute chlorpromazine produced indifference to aversive stimuli in rats.[ citation needed ]
Chlorpromazine was distributed for testing to physicians between April and August 1951. Laborit trialled the medicine on at the Val-de-Grâce military hospital in Paris, using information technology as an anaesthetic booster in intravenous doses of fifty to 100 mg on surgery patients and confirming it equally the all-time drug to date in calming and reducing shock, with patients reporting improved well existence afterwards. He also noted its hypothermic effect and suggested information technology may induce artificial hibernation. Laborit thought this would permit the torso to improve tolerate major surgery by reducing shock, a novel idea at the time. Known colloquially as "Laborit'south drug", chlorpromazine was released onto the market in 1953 by Rhône-Poulenc and given the trade name Largactil, derived from large "wide" and acti* "activity".[five]
Following on, Laborit considered whether chlorpromazine may accept a role in managing patients with astringent burns, Raynaud'south phenomenon, or psychiatric disorders. At the Villejuif Mental Infirmary in November 1951, he and Montassut administered an intravenous dose to psychiatrist Cornelia Quarti who was acting as a volunteer. Quarti noted the indifference, simply fainted upon getting up to go to the toilet, and so further testing was discontinued (orthostatic hypotension is a known side result of chlorpromazine). Despite this, Laborit continued to button for testing in psychiatric patients during early 1952. Psychiatrists were reluctant initially, but on 19 Jan 1952, it was administered (alongside pethidine, pentothal and ECT) to Jacques Lh. a 24-yr-old manic patient, who responded dramatically, and was discharged afterwards iii weeks having received 855 mg of the drug in full.[5]
Pierre Deniker had heard about Laborit'south work from his blood brother-in-law, who was a surgeon, and ordered chlorpromazine for a clinical trial at the Sainte-Anne Infirmary Center in Paris where he was Men's Service Chief.[five] Together with the Director of the hospital, Professor Jean Filibuster, they published their first clinical trial in 1952, in which they treated 38 psychotic patients with daily injections of chlorpromazine without the employ of other sedating agents.[46] The response was dramatic; treatment with chlorpromazine went beyond elementary sedation with patients showing improvements in thinking and emotional behaviour.[47] They too found that doses higher than those used past Laborit were required, giving patients 75–100 mg daily.[five]
Deniker then visited America, where the publication of their piece of work alerted the American psychiatric community that the new treatment might stand for a existent breakthrough. Heinz Lehmann of the Verdun Protestant Hospital in Montreal trialled it in 70 patients and also noted its striking furnishings, with patients' symptoms resolving later many years of unrelenting psychosis.[48] By 1954, chlorpromazine was being used in the United states to treat schizophrenia, mania, psychomotor excitement, and other psychotic disorders.[12] [49] [50] Rhône-Poulenc licensed chlorpromazine to Smith Kline & French (today's GlaxoSmithKline) in 1953. In 1955 it was approved in the United States for the treatment of emesis (vomiting). The effect of this drug in emptying psychiatric hospitals has been compared to that of penicillin and infectious diseases.[46] Merely the popularity of the drug fell from the late 1960s as newer drugs came on the scene. From chlorpromazine a number of other similar antipsychotics were developed. It also led to the discovery of antidepressants.[51]
Chlorpromazine largely replaced electroconvulsive therapy, hydrotherapy,[52] psychosurgery, and insulin shock therapy.[47] Past 1964, about 50 million people worldwide had taken it.[53] Chlorpromazine, in widespread use for l years, remains a "benchmark" drug in the treatment of schizophrenia, an effective drug although not a perfect one.[19] The relative strengths or potencies of other antipsychotics are ofttimes ranked or measured confronting chlorpromazine in aliquots of 100 mg, termed chlorpromazine equivalents or CPZE.[54]
In the motion picture: "Shutter Island", chlorpromazine is presented equally being the new medicament for psychosis treatment yet with adverse furnishings like tremors or abstinence syndrome.
Make names [edit]
Make names include Thorazine, Largactil, Hibernal, and Megaphen (sold past Bayer in W-Frg since July 1953[55]).
Veterinary use [edit]
The veterinary use of chlorpromazine has generally been superseded by use of acepromazine.[56]
Chlorpromazine may be used as an antiemetic in dogs and cats, or, less often, every bit sedative earlier anesthesia.[57] In horses, it ofttimes causes ataxia and lethargy, and is therefore seldom used.[56] [57]
It is commonly used to decrease nausea in animals that are besides young for other common anti-emetics.[ citation needed ] Information technology is also sometimes used equally a preanesthetic and musculus relaxant in cattle, swine, sheep, and goats.[ citation needed ]
The utilise of chlorpromazine in food-producing animals is not permitted in the European union, as a maximum residue limit could not be adamant following assessment past the European Medicines Bureau.[58]
Research [edit]
Chlorpromazine has tentative benefit in animals infected with Naegleria fowleri,[59] and shows antifungal and antibacterial activity in vitro.[60] [ clarification needed ]
References [edit]
- ^ "Chlorpromazine Use During Pregnancy". Drugs.com. 5 February 2020. Retrieved 21 August 2020.
- ^ "List of nationally authorised medicinal products - Agile substance: chlorpromazine : Procedure no.: PSUSA/00000715/202005" (PDF). Ema.europa.eu . Retrieved 3 March 2022.
- ^ a b c d eastward f g h i j thou l one thousand n o p q r s t u v w 10 y z "Australian Product Information – Largactil (chlorpromazine hydrochloride)" (PDF). Therapeutic Goods Administration (TGA). Sanofi Aventis Pty Ltd. 28 August 2012. Archived from the original on 30 March 2017. Retrieved 8 December 2013.
- ^ a b c d eastward f g h i j k l m "Chlorpromazine Hydrochloride". The American Social club of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 1 December 2015.
- ^ a b c d eastward f López-Muñoz, Francisco; Alamo, Cecilio; Cuenca, Eduardo; Shen, Winston W.; Clervoy, Patrick; Rubio, Gabriel (2005). "History of the discovery and clinical introduction of chlorpromazine". Annals of Clinical Psychiatry. 17 (three): 113–35. doi:10.1080/10401230591002002. PMID 16433053.
- ^ Ban, TA (Baronial 2007). "Fifty years chlorpromazine: a historical perspective". Neuropsychiatric Affliction and Handling. 3 (4): 495–500. PMC2655089. PMID 19300578.
- ^ World Health Organisation (2019). Earth Wellness Organisation model list of essential medicines: 21st list 2019. Geneva: Earth Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ López-Muñoz, F; Alamo, C; Cuenca, E; Shen, WW; Clervoy, P; Rubio, Thousand (2005). "History of the discovery and clinical introduction of chlorpromazine". Annals of Clinical Psychiatry. 17 (3): 113–35. doi:10.1080/10401230591002002. PMID 16433053.
- ^ Shorter, Edward (2005). A historical dictionary of psychiatry. New York: Oxford University Printing. p. six. ISBN9780198039235. Archived from the original on 14 Feb 2017.
- ^ Leucht, Stefan; Cipriani, Andrea; Spineli, Loukia; Mavridis, Dimitris; Örey, Deniz; Richter, Franziska; Samara, Myrto; Barbui, Corrado; Engel, Rolf R; Geddes, John R; Kissling, Werner; Stapf, Marko Paul; Lässig, Bettina; Salanti, Georgia; Davis, John M (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". The Lancet. 382 (9896): 951–62. doi:ten.1016/S0140-6736(thirteen)60733-3. PMID 23810019. S2CID 32085212.
- ^ Adams, Clive E; Awad, George A; Rathbone, John; Thornley, Ben; Soares-Weiser, Karla (6 January 2014). Cochrane Schizophrenia Group (ed.). "Chlorpromazine versus placebo for schizophrenia". Cochrane Database of Systematic Reviews. one (1): CD000284. doi:10.1002/14651858.CD000284.pub3. PMID 24395698.
- ^ a b c d east f g h i Brunton, Fifty; Chabner, B; Knollman, B (2010). Goodman and Gilman's The Pharmacological Footing of Therapeutics (12th ed.). New York: McGraw-Colina Professional. ISBN978-0-07-162442-8.
- ^ American Society of Health-Organization Pharmacists (ane November 2008). "Chlorpromazine". PubMed Health. National Center for Biotechnology Information. Archived from the original on half-dozen July 2010.
- ^ Breitbart, West; Marotta, R; Platt, MM; et al. (Feb 1996). "A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients". The American Journal of Psychiatry. 153 (2): 231–37. doi:x.1176/ajp.153.2.231. PMID 8561204.
- ^ a b Chlorpromazine. Martindale: The Consummate Drug Reference. London: Pharmaceutical Printing. thirty Jan 2013. Retrieved viii December 2013.
- ^ Logan, Peter; Lewis, David (Apr 2007). "Chlorpromazine in Migraine". Emergency Medicine Journal. 24 (4): 297–300. doi:10.1136/emj.2007.047860. PMC2658244. PMID 17384391.
- ^ Richter, PA; Burk, MP (July–August 1992). "The potentiation of narcotic analgesics with phenothiazines". The Journal of Foot Surgery. 31 (iv): 378–80. PMID 1357024.
- ^ "Propaphenin, Medicine and Disease information". EPG Online. 14 July 2001. Archived from the original on 2 Dec 2013. Retrieved 26 November 2013.
- ^ a b c Adams CE, Awad K, Rathbone J, Thornley B, Soares-Weiser Chiliad (2014). "Chlorpromazine versus placebo for schizophrenia". Cochrane Database of Systematic Reviews. i (1): CD000284. doi:10.1002/14651858.CD000284.pub3. PMID 24395698. Archived from the original on 1 October 2015.
- ^ Pisani, F; Oteri, K; Costa, C; Di Raimondo, G; Di Perri, R (2002). "Effects of psychotropic drugs on seizure threshold". Drug Prophylactic. 25 (2): 91–110. doi:ten.2165/00002018-200225020-00004. PMID 11888352. S2CID 25290793.
- ^ Leucht C, Kitzmantel M, Chua Fifty, Kane J, Leucht S (2008). Leucht C (ed.). "Haloperidol versus chlorpromazine for schizophrenia". Cochrane Database of Systematic Reviews (1): CD004278. doi:10.1002/14651858.CD004278.pub2. PMID 18254045.
- ^ Leucht S, Wahlbeck Chiliad, Hamann J, Kissling W (May 2003). "New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis". Lancet. 361 (9369): 1581–89. doi:10.1016/S0140-6736(03)13306-five. PMID 12747876. S2CID 40851775.
- ^ Thomas D; Wu K; Kathöfer S; et al. (June 2003). "The antipsychotic drug chlorpromazine inhibits HERG potassium channels". British Periodical of Pharmacology. 139 (3): 567–74. doi:10.1038/sj.bjp.0705283. PMC1573882. PMID 12788816.
- ^ Articulation Formulary Commission, BMJ, ed. (March 2009). "4.two.1". British National Formulary (57 ed.). United kingdom: Purple Pharmaceutical Society of Great United kingdom. p. 192. ISBN978-0-85369-845-half dozen.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the adventure of acute withdrawal syndromes or rapid relapse.
- ^ a b c d e Haddad, Peter; Haddad, Peter Thousand.; Dursun, Serdar; Deakin, Neb (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. pp. 207–16. ISBN9780198527480.
- ^ Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–thirteen. doi:10.1111/j.1600-0447.2006.00787.ten. PMID 16774655. S2CID 6267180.
- ^ Sacchetti, Emilio; Vita, Antonio; Siracusano, Alberto; Fleischhacker, Wolfgang (2013). Adherence to Antipsychotics in Schizophrenia. Springer Scientific discipline & Business Media. p. 85. ISBN9788847026797.
- ^ "Chlorpromazine Hydrochloride 100mg/5ml Oral Syrup – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Rosemont Pharmaceuticals Express. six August 2013. Archived from the original on xi December 2013. Retrieved 8 Dec 2013.
- ^ Dahl SG, Strandjord RE (Apr 1977). "Pharmacokinetics of chlorpromazine after unmarried and chronic dosage". Clinical Pharmacology and Therapeutics. 21 (4): 437–48. doi:10.1002/cpt1977214437. PMID 849674. S2CID 6645825.
- ^ a b Yeung PK, Hubbard JW, Korchinski ED, Midha KK (1993). "Pharmacokinetics of chlorpromazine and fundamental metabolites". European Journal of Clinical Pharmacology. 45 (vi): 563–69. doi:10.1007/BF00315316. PMID 8157044. S2CID 6410850.
- ^ Maheux, Jérôme; Éthier, Isabelle; Rouillard, Claude; Lévesque, Daniel (April 2005). "Induction Patterns of Transcription Factors of the Nur Family ( Nurr1, Nur77, and Nor -1) by Typical and Atypical Antipsychotics in the Mouse Brain: Implication for Their Mechanism of Action". Journal of Pharmacology and Experimental Therapeutics. 313 (1): 460–73. doi:x.1124/jpet.104.080184. hdl:20.500.11794/17025. ISSN 0022-3565. PMID 15615863. S2CID 1436507.
- ^ a b c d eastward f g h i j k l m n o p q r s t "Chlorpromazine". PDSP Database.
- ^ Gillman, P.Chiliad. (October 2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–41. doi:ten.1093/bja/aei210. PMID 16051647.
- ^ Kroeze, Wesley M; Hufeisen, Sandra J; Popadak, Beth A; Renock, Sean Chiliad; Steinberg, SeAnna; Ernsberger, Paul; Jayathilake, Karu; Meltzer, Herbert Y; Roth, Bryan L (March 2003). "H1-Histamine Receptor Analogousness Predicts Curt-Term Weight Gain for Typical and Atypical Antipsychotic Drugs". Neuropsychopharmacology. 28 (3): 519–26. doi:10.1038/sj.npp.1300027. ISSN 0893-133X. PMID 12629531.
- ^ a b c d e Silvestre, J.Due south.; Prous, J. (2005). "Enquiry on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce blazon 2 diabetes". Methods and Findings in Experimental and Clinical Pharmacology. 27 (5): 289–304. doi:10.1358/mf.2005.27.5.908643. ISSN 0379-0355. PMID 16082416.
- ^ a b c von Coburg, Y.; Kottke, T.; Weizel, L.; Ligneau, Ten.; Stark, H. (Jan 2009). "Potential utility of histamine H3 receptor antagonist pharmacophore in antipsychotics". Bioorganic & Medicinal Chemistry Letters. nineteen (2): 538–42. doi:10.1016/j.bmcl.2008.09.012. PMID 19091563.
- ^ Girault J, Greengard P (2004). "The neurobiology of dopamine signaling". Curvation Neurol. 61 (5): 641–44. doi:10.1001/archneur.61.5.641. PMID 15148138.
- ^ a b McKim, William A. (2007). Drugs and beliefs: an introduction to behavioral pharmacology (6th ed.). Upper Saddle River, New Jersey: Prentice Hall. p. 416. ISBN978-0-thirteen-219788-five.
- ^ Peroutka SJ, Synder SH (December 1980). "Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency". The American Journal of Psychiatry. 137 (12): 1518–22. doi:10.1176/ajp.137.12.1518. PMID 6108081.
- ^ Lidsky TI, Yablonsky-Change E, Zuck LG, Banerjee SP (August 1997). "Antipsychotic drug effects on glutamatergic activity". Brain Research. 764 (one–2): 46–52. doi:ten.1016/S0006-8993(97)00423-X. PMID 9295192. S2CID 37454572.
- ^ Kornhuber J, Muehlbacher One thousand, Trapp South, Pechmann S, Friedl A, Reichel One thousand, Mühle C, Terfloth L, Groemer T, Spitzer 1000, Liedl K, Gulbins E, Tripal P (2011). Riezman H (ed.). "Identification of novel functional inhibitors of acid sphingomyelinase". PLOS Ane. 6 (8): e23852. Bibcode:2011PLoSO...623852K. doi:10.1371/journal.pone.0023852. PMC3166082. PMID 21909365.
- ^ Falkai, P; Vogeley G (April 2000). "The chances of new atypical substances". Fortschritte der Neurologie-Psychiatrie. biopsychiatry.com. 68 Suppl 1: S32–37. PMID 10907611. Archived from the original on 24 July 2010. Retrieved 6 July 2010.
- ^ "Thorazine advertisement". Smith Kline & French. c. 1963.
When the patient lashes out against 'them' – Thorazine (brand of chlorpromazine) rapidly puts an end to his violent outburst. 'Thorazine' is especially constructive when the psychotic episode is triggered by delusions or hallucinations. At the commencement of treatment, Thorazine's combination of antipsychotic and sedative furnishings provides both emotional and physical calming. Assaultive or destructive behavior is rapidly controlled. As therapy continues, the initial sedative effect gradually disappears. Simply the antipsychotic effect continues, helping to dispel or modify delusions, hallucinations and defoliation, while keeping the patient calm and approachable. Smith Kline and French Laboratories
- ^ Healy, David (2004). "Explorations in a new world". The creation of psychopharmacology. Harvard Academy Press. p. 77. ISBN978-0-674-01599-nine. Archived from the original on viii September 2017. Retrieved 26 November 2013.
- ^ Healy, David (2004). "Explorations in a new world". The cosmos of psychopharmacology. Harvard University Press. p. 80. ISBN978-0-674-01599-9.
- ^ a b Turner, T (Jan 2007). "Chlorpromazine: unlocking psychosis". BMJ. 334 (Suppl i): s7. doi:10.1136/bmj.39034.609074.94. PMID 17204765. S2CID 33739419.
- ^ a b Healy, David (2004). The Cosmos of Psychopharmacology. Harvard University Press. pp. 37–73. ISBN978-0-674-01599-ix. Archived from the original on 8 September 2017. Retrieved 26 November 2013.
- ^ Dronsfield2006-05-01T00:00:00+01:00, Alan. "Chlorpromazine - unlocks the saylum". RSC Education . Retrieved 13 Jan 2022.
- ^ Long, James W. (1992). The Essential guide to prescription drugs. New York: HarperPerennial. pp. 321–25. ISBN978-0-06-271534-0.
- ^ Reines, Brandon P (1990). "The Human relationship Betwixt Laboratory and Clinical Studies in Psychopharmacologic Discovery". Perspectives on Medical Inquiry. Medical Research Modernization Society. 2. Archived from the original on 7 September 2015. Retrieved 26 November 2013.
- ^ Healy, David (2004). "Introduction". The Creation of Psychopharmacology. Harvard Academy Press. p. 2. ISBN9780674015999. Archived from the original on eight September 2017. Retrieved 26 November 2013.
- ^ Healy, David (2000). "Psychopharmacology and the Government of the Cocky" (PDF). davidhealy.org. Archived from the original (PDF) on vi October 2014. Retrieved xx July 2015.
- ^ "Drug for treating schizophrenia identified". PBS.org. WGBH-Television. Archived from the original on 18 September 2009. Retrieved 7 July 2010.
- ^ Yorston, K. (2000). "Chlorpromazine equivalents and percentage of British National Formulary maximum recommended dose in patients receiving loftier-dose antipsychotics". Psychiatric Bulletin. 24 (4): 130–32. doi:10.1192/pb.24.4.130.
- ^ Bangen, Hans (1992). Geschichte der medikamentösen Therapie der Schizophrenie. Verlag für Wissenschaft und Bildung. p. 98. ISBN 3-927-408-82-four.
- ^ a b Plumb, Donald C. (2015). Plumb'south Veterinarian Drug Handbook (8th ed.). John Wiley & Sons. ISBN978-1118911921.
- ^ a b Posner, Lysa A.; Burns, Patrick (2009). "Chapter xiii: Sedative agents: tranquilizers, alpha-2 agonists, and related agents". In Riviere, Jim E.; Papich, Mark Thousand.; Adams, Richard H. (eds.). Veterinary pharmacology and therapeutics (9 ed.). Ames, Iowa: Wiley-Blackwell. pp. 337–fourscore. ISBN9780813820613.
- ^ "Chlorpromazine: summary report" (PDF). European Medicines Agency. Committee for Veterinarian Medicinal Products. June 1996. Archived (PDF) from the original on xviii January 2017. Retrieved 17 January 2017.
- ^ Kim, JH; Jung, SY; Lee, YJ; Song, KJ; Kwon, D; Kim, Yard; Park, S; Im, KI; Shin, HJ (November 2008). "Effect of therapeutic chemical agents in vitro and on experimental meningoencephalitis due to Naegleria fowleri". Antimicrobial Agents and Chemotherapy. 52 (11): 4010–4016. doi:10.1128/AAC.00197-08. PMC2573150. PMID 18765686.
- ^ Afeltra, J.; Verweij, P. Eastward. (1 July 2003). "Antifungal Activity of Nonantifungal Drugs". European Journal of Clinical Microbiology & Infectious Diseases. Springer Nature. 22 (vii): 397–407. doi:10.1007/s10096-003-0947-10. ISSN 0934-9723. PMID 12884072. S2CID 10489462.
External links [edit]
- "Chlorpromazine". Drug Data Portal. U.S. National Library of Medicine.
- "Chlorpromazine hydrochloride". Drug Information Portal. U.S. National Library of Medicine.
Source: https://en.wikipedia.org/wiki/Chlorpromazine
Posted by: boyeriveresel.blogspot.com

0 Response to "How Much Money Does Glazosmithkline Make On Thorazine"
Post a Comment